Since the state of emergency due to the COVID-19 crisis, the FAMES Lab directed its efforts toward the rapid fabrication of medical equipment for caretakers at IU Health, Eskenazi Health, and Deaconess Health as announced previously in our news. Among its multple efforts, Louis van der Elst and Merve Gokce Kurtoglu concentrated their efforts on 3D printing nasopharyngeal swabs which lead to a publication of its engineering design and fabrication process. This research is specifically focused at mass rapid fabrication of nasopharyngeal swabs during the beginning crisis stage of a pandemic. An interview of Prof. Alexander Gumennik on this research was conducted by Ken Birkoff from the Luddy School of Informatics, Computing, and Engineering, is accessible here.
This paper focuses on different considerations and approaches to swab design, spurred by the present COVID-19 pandemic. It argues that, given the urgent need for widespread testing to make the pandemic more manageable, using stereolithography to 3D print testing swabs offers the safest, most efficient method to fulfill the short-term supply chain resilience needs. In addition to offering a review of current and past designs, the paper also provides innovative effective design alternatives. This two-fold approach to understanding swab design enables its authors to offer constructive guidelines for those looking to design their own swabs.
The paper provides an overview of previously conducted comparisons of different swab materials, testing sites, and design compositions. It then discusses the current attempts at 3D printed swab designs that have emerged as a result of this pandemic. Next, is discussed stereolithography and dental photopolymers as together being well suited for biomedical device fabrication. Finally, it offers the design specifications and results of the FAMES Lab’s own designs. Comparing the efficacy of each swab design, and compounding these results with the specific demands of COVID-19 testing, we provide a set of guidelines and constraints any swab design should follow.
This paper serves as a means to bridge the gap between the current demands in testing supplies and the massive capabilities of 3D printing bioengineering labs around the world. The design guidelines we provide are based on existing biomedical standards and introduce key requirements for addtive manufacturers to print their own nsopharyngeal swabs in terms of safety, efficiency, mass fabrication, and more. This paper also sets the stage for optimizing the implementation process of the rapid design and fabrication of medical devices for local supply chain resilience, until global solutions are made available.
This paper offers the following novel contributions:
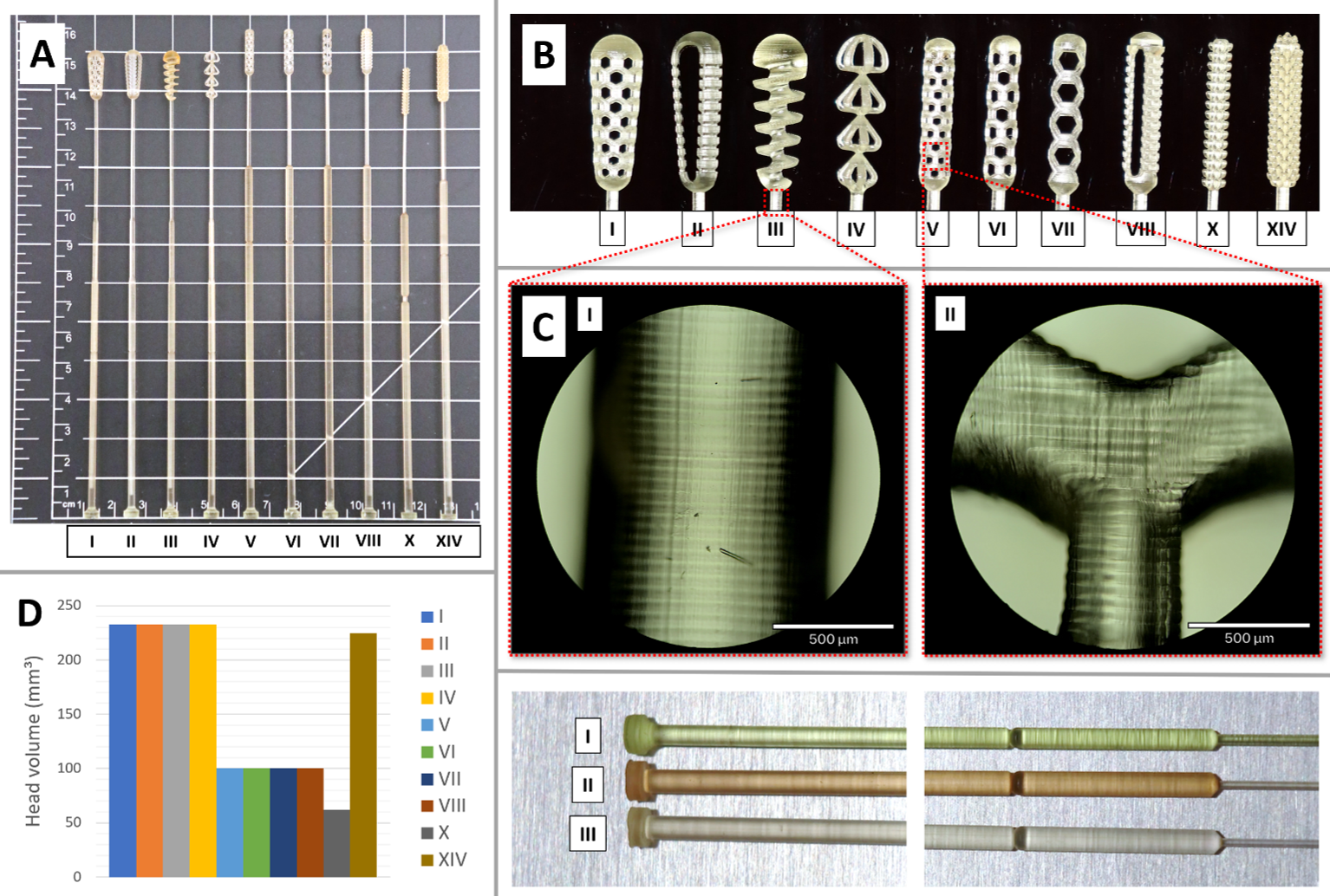
- A comparison of the retention and comfort performance of a range of novel designs of 3D printed swabs to the flocked-head swab, commonly used in clinical environment.
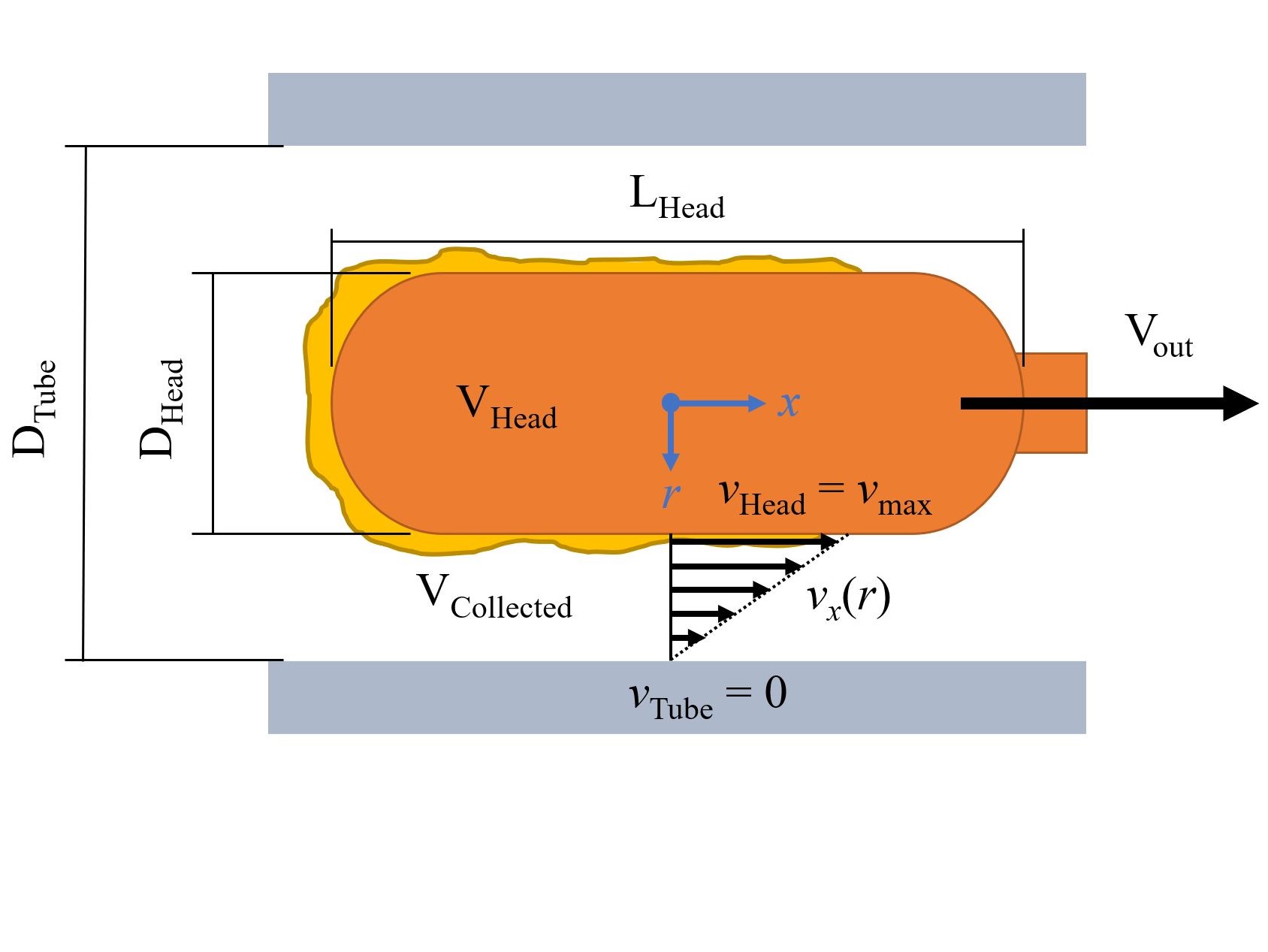
- A determination of the parameters governing the performance of 3D printed swabs in terms of mucus retention, which include the volume of the head, porosity density of the head and void fraction, as well as pore geometry.
- An ideal “bubble-wand” shape design, which maximizes the empty volume of the head, the pore size, with optimized geometry for maximized surface tension.
- Suggestions for mechanically functional designs of the swab head for enhanced functionality, such as corkscrew head design with chirality dependent retention, and a head designed to have negative Poisson ratio to axial compressive stress, for an increased patient comfort when inserted into, and maximized sample collection when extracted from the nasal cavity.
- Demonstrations of how the shaft design of a swab can be optimized for ease of snapping at the score, which delineates the sacrificial shaft from the sample part of the swab, allowing it to be optimized for efficient operation by healthcare professionals.
- A demonstrations of a protocol for preclinical trials of swabs based on custom developed artificial mucus and 3D printed model of the nasal cavity, providing a non-invasive in-vitro characterization methodology for swabs.
The article is open-access and can be read in Advanced Engineering Materials here.