The FAMES Lab at Indiana University has received a new patent, US12102734B2 titled "Methods for Creating Three-Dimensional Biosynthetic Tissue." Its inventors, Alexander Gumennik, Louis van der Elst, and Merve Gokce, seek to advance biomedical innovation closer to creating functional tissues that can be used in medical treatments, improving clinical outcomes and advancing our understanding of human diseases.
Recent advancements in bioengineering offer potential solutions for creating functional tissues and organs to address medical needs, from wound healing to organ regeneration. Despite significant progress in bioprinting, a key challenge in tissue engineering has been the development of tissues that closely mimic the body’s natural structure and processes.
A major difficulty in tissue engineering is vascularization—the network of blood vessels that supply oxygen and nutrients while removing waste. While natural tissues benefit from a complex microvascular network, engineered tissues often lack this vital system, limiting their viability and functionality. Most current tissue fabrication methods rely on diffusion to provide oxygen and nutrients, but this process is only effective over short distances, restricting the thickness of the tissues that can be created.
Additionally, the absence of features like the extracellular matrix (ECM) with complex fiber structures and adaptive signaling further hinders tissue maturation. As a result, many engineered tissues struggle to self-organize and function properly once implanted. Real-time monitoring of metabolic processes and local conditions within these tissues has also been a challenge, making it difficult to adjust conditions to promote healthy development.
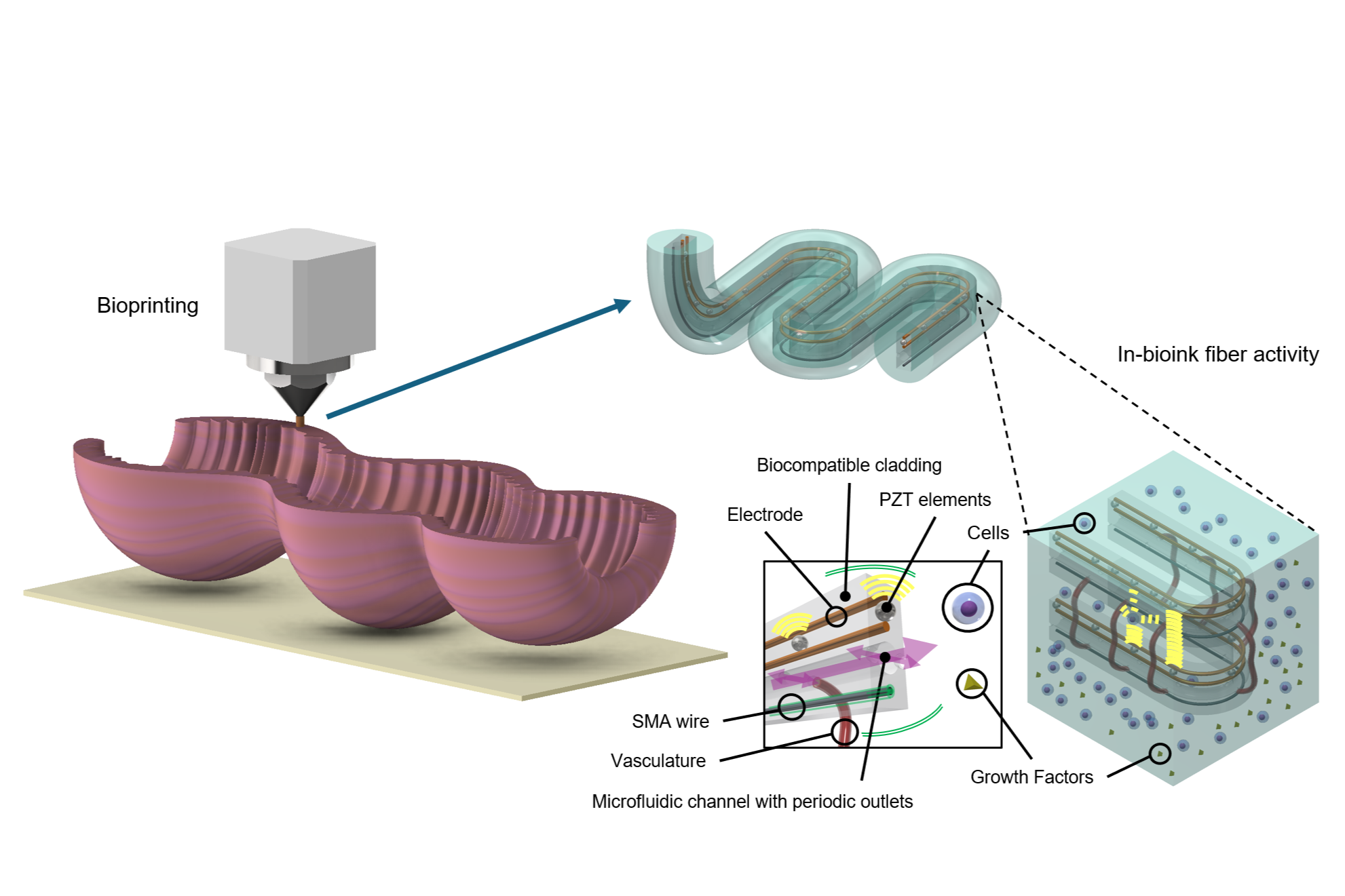
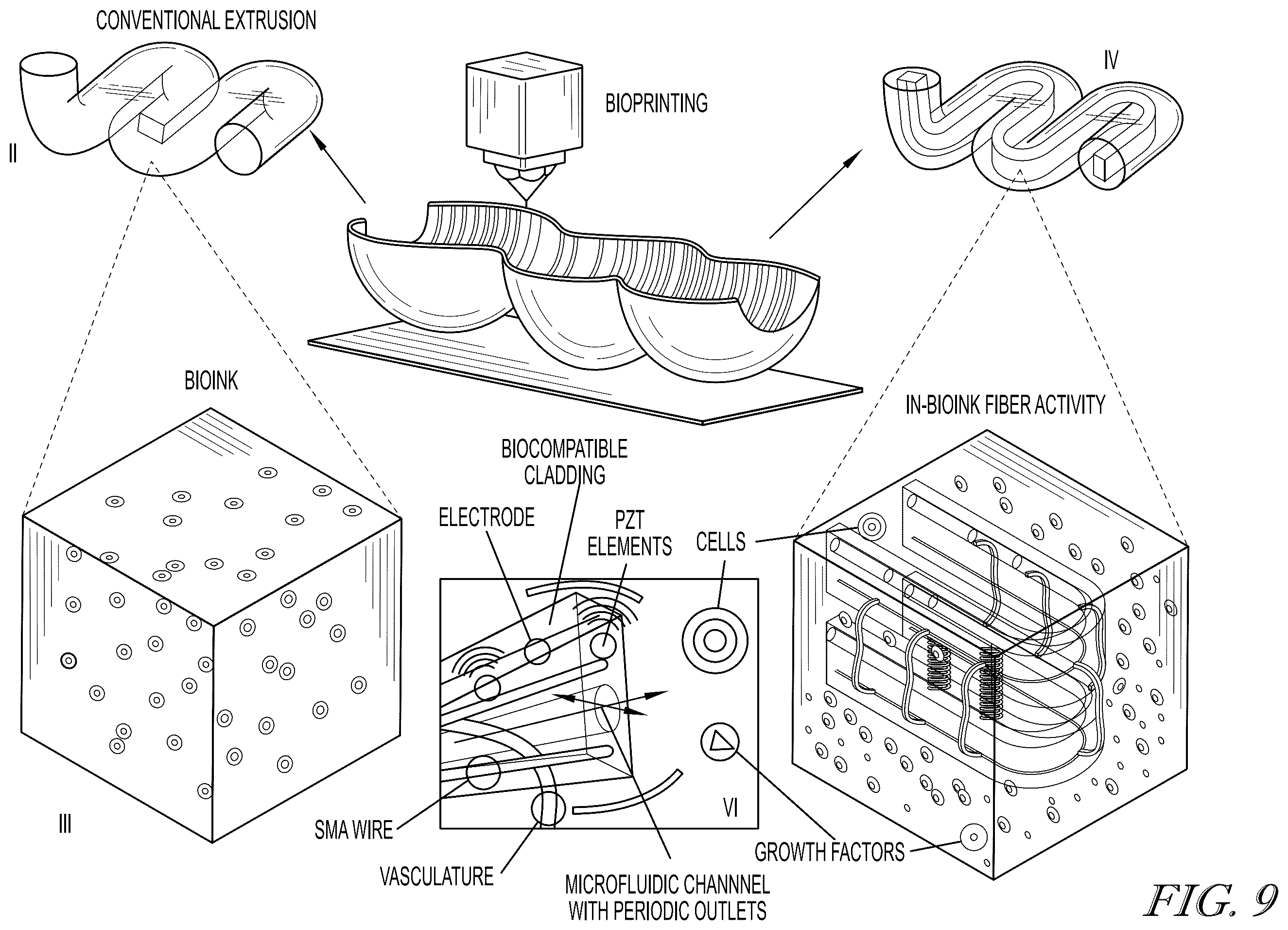
A recent development addresses these challenges by integrating real-time monitoring and manipulation of local tissue conditions to support proper maturation. This system aims to create bioengineered tissues with functional structures, such as blood vessels, nerves, and muscle tissue. The approach involves a bioink-coated microstructured fiber that serves as a scaffold for cell growth, with microfluidic channels designed to simulate blood flow.
Sensors embedded in the fibers allow continuous monitoring of the tissue’s metabolic processes, including temperature, chemical composition, and oxygen levels. This feedback enables more precise control over tissue development and can be adjusted to optimize cell viability and the formation of the extracellular matrix. These innovations may lead to the creation of functional tissues and organs for applications in prosthetics, organ regeneration, and treatment of inborn defects.
In addition, these advancements could support drug testing and cancer research, offering a more accurate platform for assessing toxicity without relying on animal models. They could also improve cancer research by providing more reliable models of tumor growth and responses to treatment.
These bioengineering developments could contribute to advancements in regenerative medicine, offering potential solutions for patients in need of tissue replacement or treatments for degenerative diseases. By enabling the creation of realistic, three-dimensional tissues and organs, these technologies represent a significant step forward in the development of functional tissues for a variety of medical applications.